Monoclonal Antibody Treatment For Covid
Monoclonal antibody therapy is used to fight COVID early on but over at Orlando Health their emergency department ED isnt able to treat patients with it. If you think you may qualify for mAb treatments and want to ask about getting treatment contact your healthcare provider.
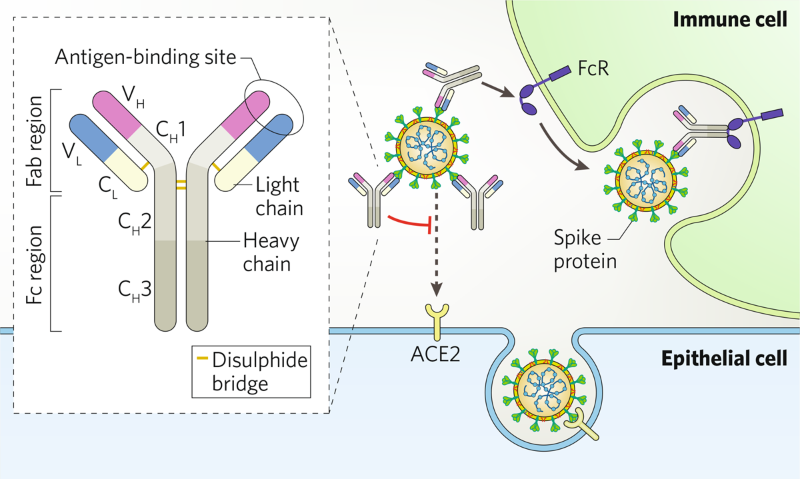
Hunting For Antibodies To Combat Covid 19
Currently two different types of monoclonal antibody treatments have been approved.
Monoclonal antibody treatment for covid. SALT LAKE CITY ABC4 In a new study researchers from Intermountain Healthcare have found that treating high-risk COVID-19 patients with a monoclonal antibody treatment reduced severe illness and hospitalizations by more than 50 and saved many patients from dying due to complications from the virus. Monoclonal antibody treatment available for early COVID-19 at Stanford Health Care An infusion of monoclonal antibodies can ease COVID-19 symptoms and reduce complications in recently diagnosed non-hospitalized people at high risk. Health care providers may recommend these treatments.
President Donald Trump in. MAb treatment is most effective when received soon after COVID-19 symptoms begin so it is important to get tested right away. Thats why mAb treatment may help patients who are at high risk for severe symptoms or hospitalization.
Experts turn to antibody treatment following swarm of breakthrough COVID-19 infections With high-risk breakthrough COVID infections monoclonal antibodies may. UPMC received two monoclonal antibody infusion treatment products. As Healthcare providers continue to fight the COVID-19 pandemic effective treatments are needed to reduce transmission rate hospitalizations and the overall burden of COVID-19 on the United States health care system.
Treatment regimen is one dose. Monoclonal antibody treatment for COVID-19 has received emergency use authorization by the Food and Drug Administration FDA as an investigational medicine used for the treatment of COVID-19 patients at high-risk of developing severe illness. For example the FDA has issued EUAs for several monoclonal antibody treatments for COVID-19 for the treatment of mild or moderate COVID-19 in.
Depending on age medical history and length of time symptomatic monoclonal antibody mAb treatment. One of these treatments is a combination of the drugs bamlanivimab and etesevimab administered together while the other is a combination of the drugs casirivimab and imdevimab. FDA authorizes REGEN-COV monoclonal antibody therapy for post-exposure prophylaxis prevention for COVID-19.
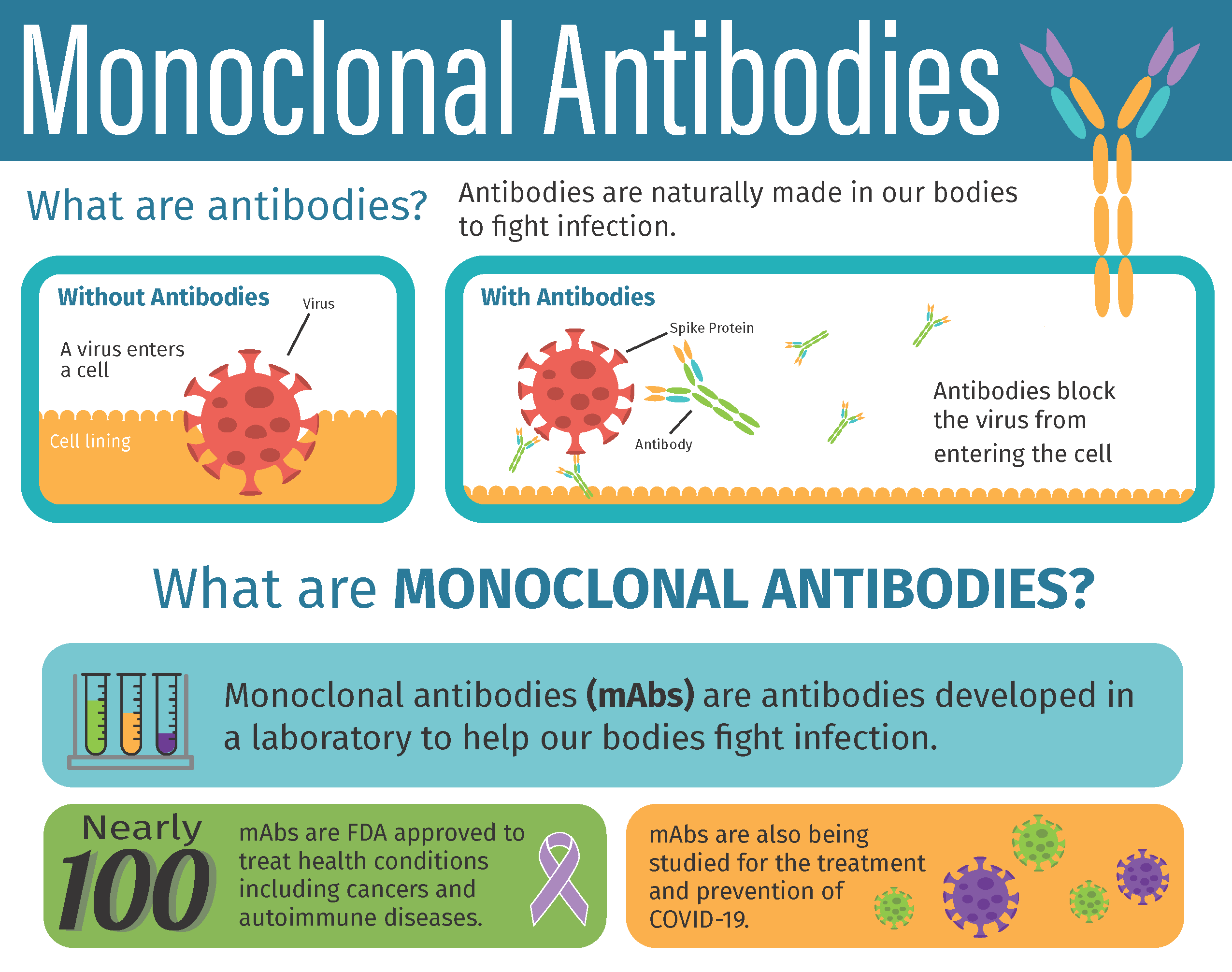
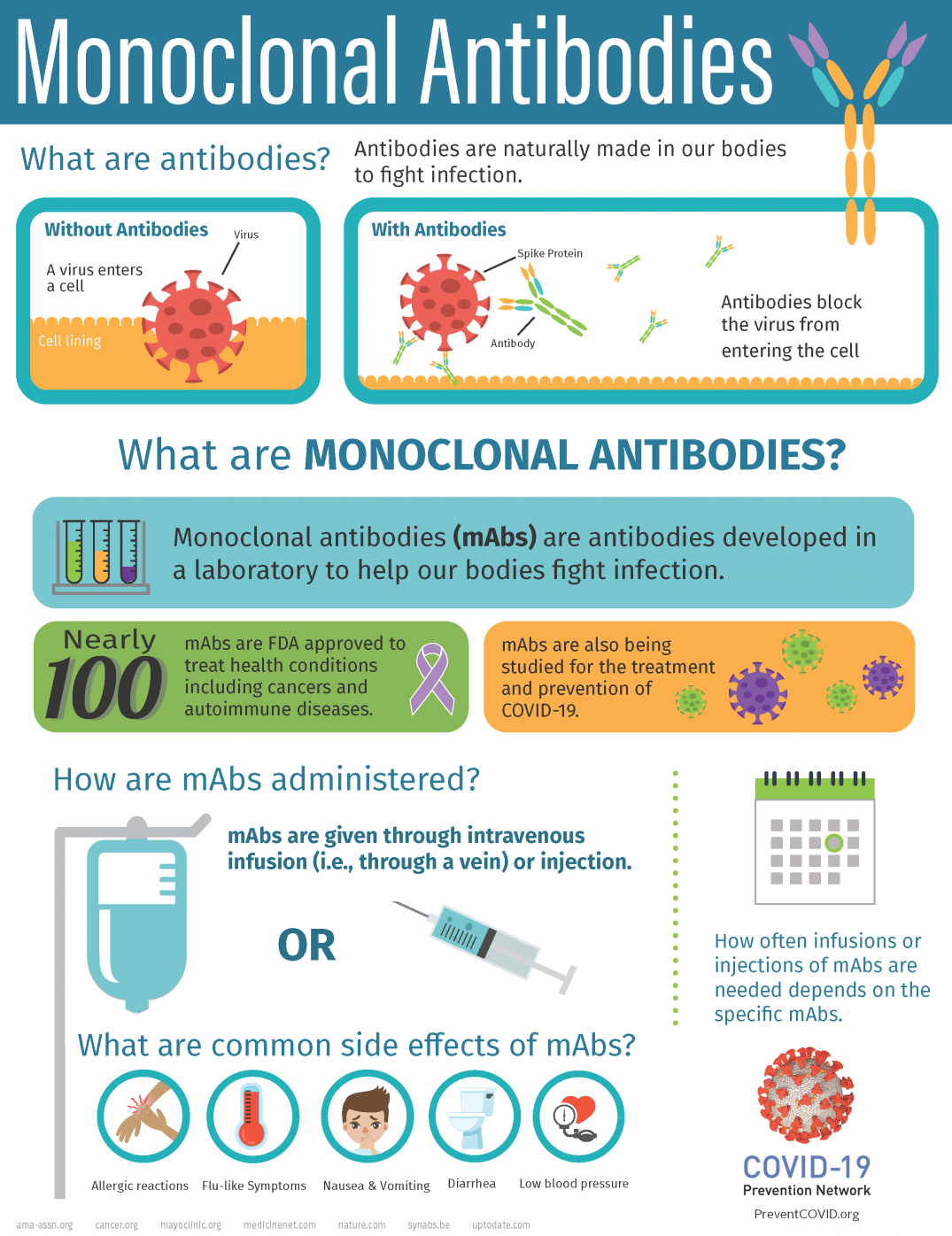
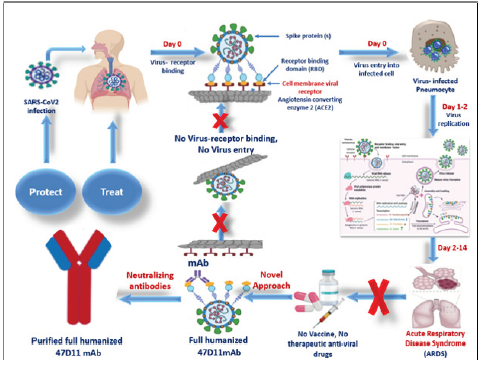
Monoclonal antibodies or mAbs are made in a laboratory to fight a particular infectionin this case the virus that causes COVID-19and are given to patients directly with an infusion or a shot. Some early evidence suggests that mAb treatment can reduce the amount of the SARS-CoV-2 virus the virus that causes COVID-19 in a persons system. Monoclonal antibodies are laboratory-made proteins designed to mimic the bodys ability to fight off viruses and pathogens.
COVID-19 Monoclonal Antibody Therapy Resource Center. Now people can refer themselves. Its called monoclonal antibody mAb treatment.
Monoclonal antibodies are a treatment for COVID-19 approved under an FDA Emergency Use Authorization EUA. Treatment of monoclonal antibodies for COVID-19 should be given as soon as possible after a positive COVID-19 test and within 10 days of symptom onset. Bamlanivimab and casirivimab plus imdevimab.
In 2020 the FDA authorized several different monoclonal antibodies to treat COVID-19. Monoclonal Antibodies in COVID-19. For people at high risk of getting very sick from COVID-19 monoclonal antibody treatment given early can significantly reduce the risk of progressing to severe COVID-19 disease and needing hospitalization.
This is the first time an injectable coronavirus antibody treatment has been approved for use as a prevention of Covid after someone has been exposed to the virus. MAb treatment can lower the amount of virus in your body reduce symptoms and help avoid a trip to the hospital. Both Eli Lillys monoclonal antibody and a similar two-antibody cocktail from Regeneron Pharmaceuticalsfamously used to treat former US.
Monoclonal antibodies mAbs are immune system proteins developed from a single cell lineage that demonstrate a high affinity for their target cell. Prophylaxis with REGEN-COV is not a substitute for vaccination. These are lab-designed antibodies that can detect the virus and help your immune system clear it.
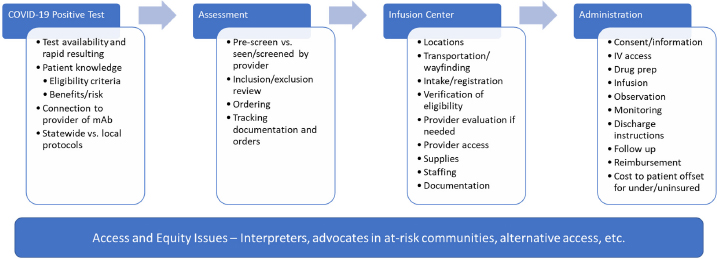
Patients should be clinically monitored during and after infusion or injections for at least one hour. Monoclonal antibody mAb treatment is for people who have tested positive for COVID-19 and are not sick enough to be in the hospital. The antibody treatment must be given to a.
Monoclonal antibodies were first developed by Köhler and Milstein in 1975 using hybridoma technology. The treatment can also shorten the duration of. You can learn more about mAb treatments on our page Monoclonal Antibodies for High-Risk COVID-19 Positive Patients.
This amount is known as viral load. Patients should continue to self-isolate and use infection control measures according to CDC guidelines.
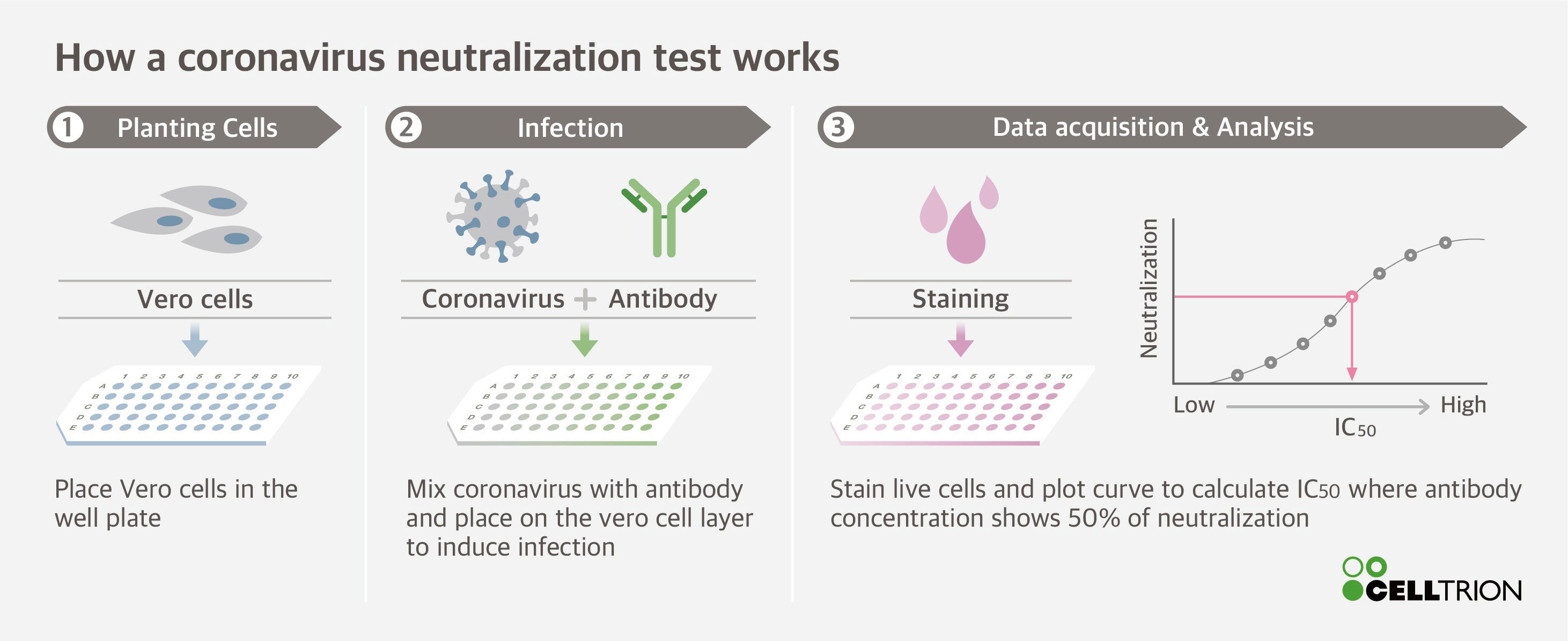
Celltrion Completes Neutralisation Test On Candidate Monoclonal Antibodies Mabs For Covid 19 Antiviral Antibody Treatment Business Wire
Monoclonal Antibodies And Other Novel Therapeutics In Covid 19 Treatment Mayo Clinic School Of Continuous Professional Development
Pages Monoclonal Antibody Treatment For Covid 19
Rapid Expert Consultation On Allocating Covid 19 Monoclonal Antibody Therapies And Other Novel Therapeutics January 29 2021 Rapid Expert Consultation On Allocating Covid 19 Monoclonal Antibody Therapies And Other Novel Therapeutics January 29

What Is Monoclonal Antibody Therapy Who Is Eligible To Receive It Houston Methodist On Health

Monoclonal Antibody Treatment Idph
How Monoclonal Antibodies Work Covid 19 Prevention Network

What Are Monoclonal Antibodies And Can They Treat Covid 19 Iav

Monoclonal Antibody Treatment Wise Health System

An Update On Covid 19 Treatments Monoclonal Antibodies Convalescent Plasma And Other Promising Developments Covid 19 Johns Hopkins Bloomberg School Of Public Health

Antibody Treatments For Covid 19 Are Worth The Effort Doctors Say Shots Health News Npr
Covid 19 Immunotherapy Novel Humanized 47d11 Monoclonal Antibody
.jpg)
Monoclonal Antibody Treatment Tucson Arizona Az Tucson Medical Center
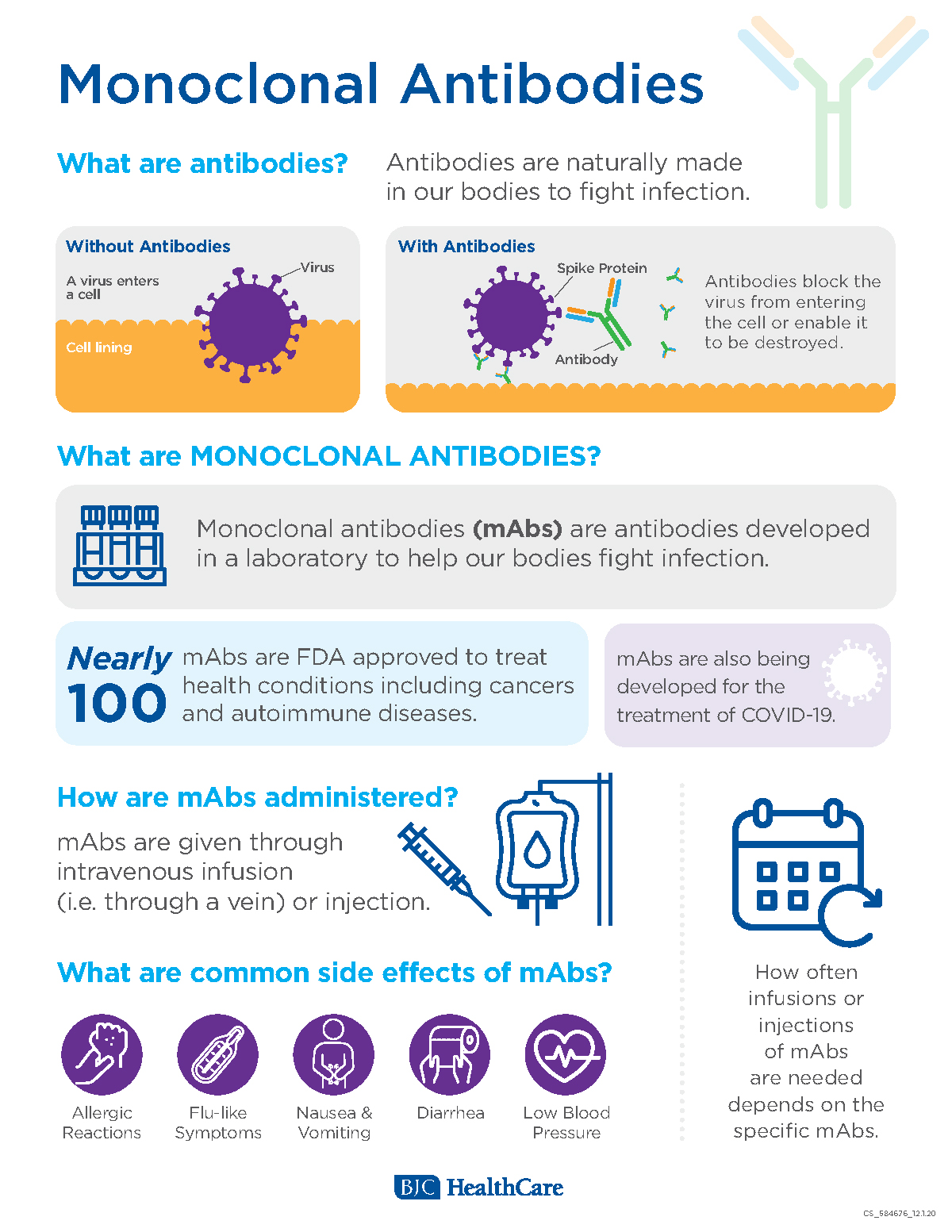
Bjc Healthcare Coronavirus Mab For Covid 1
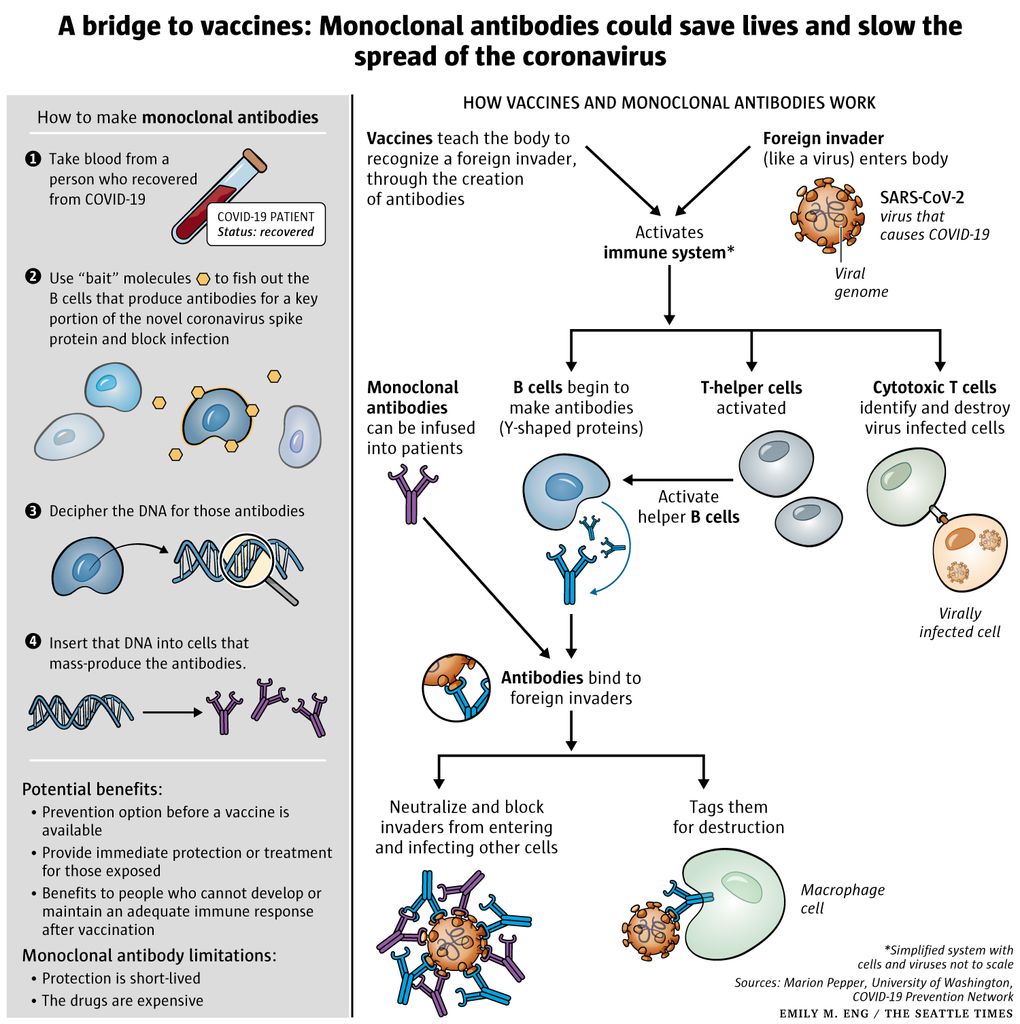
Monoclonal Antibody Therapy In Covid 19 Treatment Northwest Asthma Allergy Center

Monoclonal Antibody Cocktail Authorized For Post Covid Exposure Medpage Today
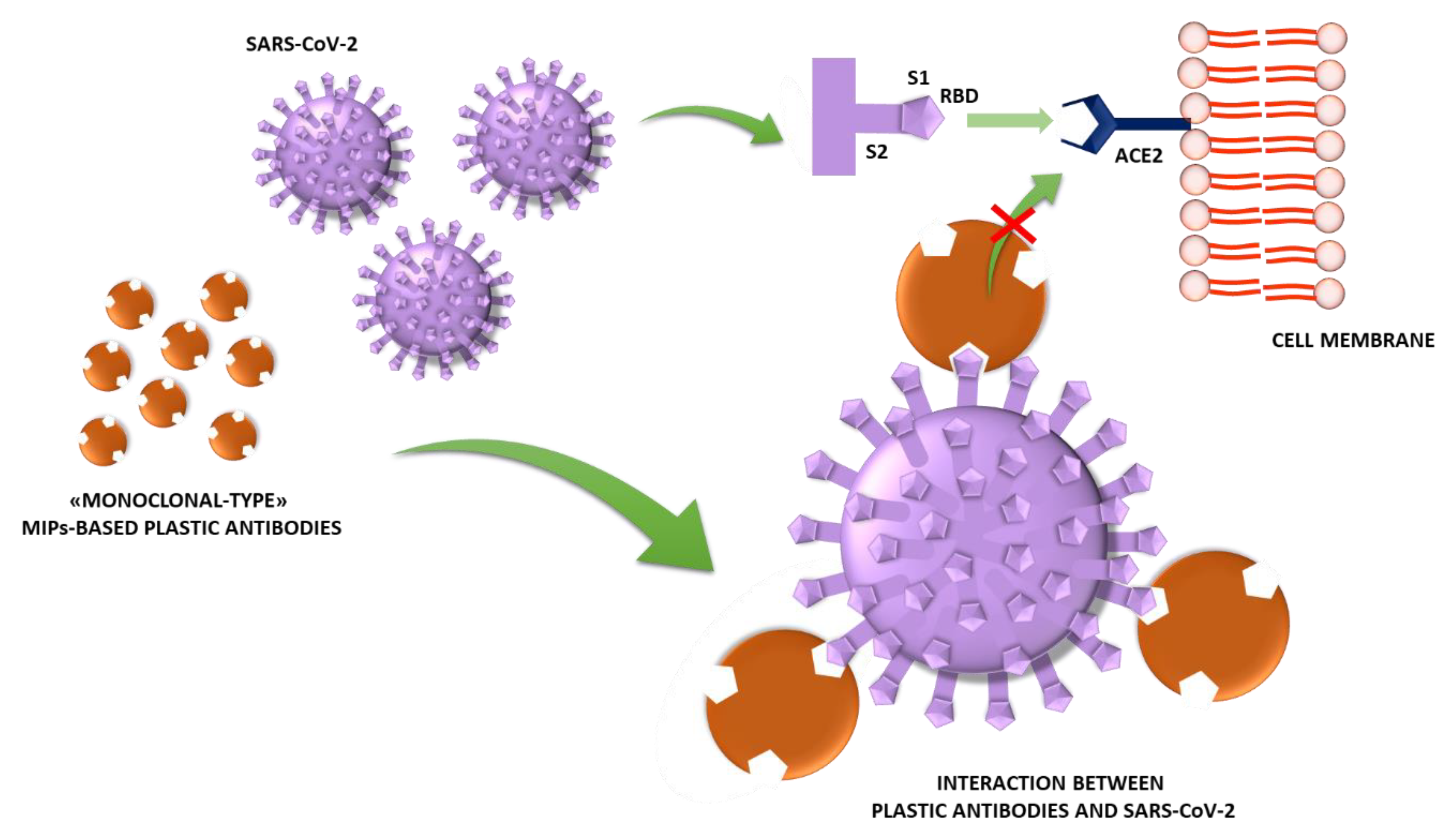
Jfb Free Full Text Monoclonal Type Plastic Antibodies For Covid 19 Treatment What Is The Idea Html
Johns Hopkins Helps Administer Covid 19 Antibody Therapy At Baltimore Convention Center Field Hospital Johns Hopkins Medicine

Post a Comment for "Monoclonal Antibody Treatment For Covid"