Monoclonal Antibody Treatment Los Angeles
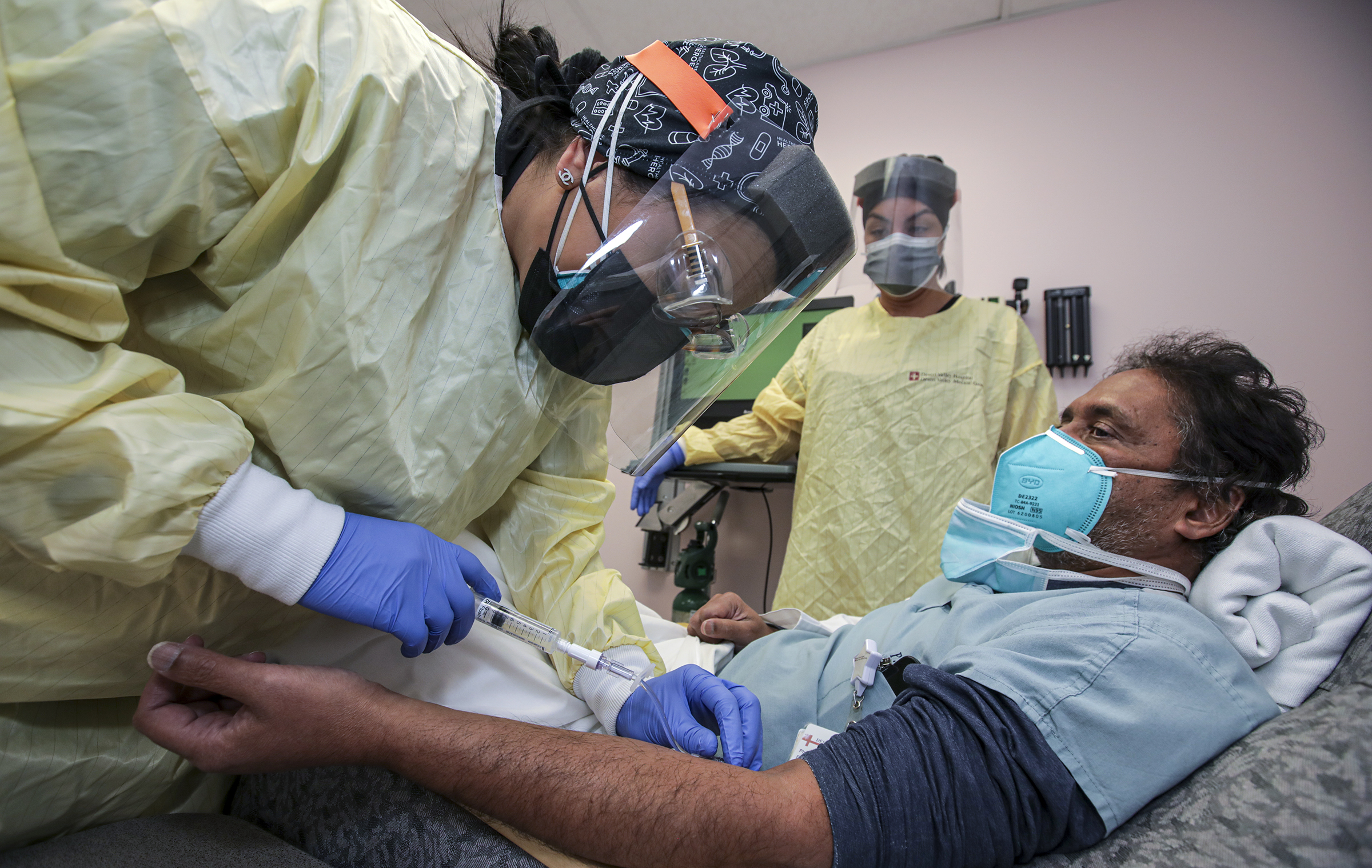
Many Emergency Departments and Infusion Clinics offer monoclonal antibody. A patient receives a monoclonal antibody infusion at Desert Valley Hospital in Victorville.
Locating Antibody Treatments For Covid 19 Can Be A Treasure Hunt Shots Health News Npr
Monoclonal Antibody Treatment is accessible in many locations throughout Los Angeles County for eligible patients.

Monoclonal antibody treatment los angeles. The Covid-19 treatments that have an emergency use authorization are made by Regeneron GlaxoSmithKline and Eli Lilly. Monoclonal antibodies or mAbs are made in a laboratory to fight a particular infectionin this case SARS-CoV-2and are given to patients directly with an infusion. Monoclonal antibodies are lab-developed antibodies that mimic those naturally produced by the immune system in response to infection.
Getty Images The nationwide phase 3 clinical trial is for a monoclonal antibody drug known as. A drive-thru COVID-19 testing site in South Los Angeles on Nov. You may occasionally receive promotional content from the Los Angeles Times.
Thats why mAb treatment may help patients who are at high risk for severe symptoms or having to be hospitalized. The two monoclonal antibody treatments at the forefront of COVID-19 studies are Eli Lillys product bamlanivimab and a cocktail from Regeneron Pharmaceuticals that Trump received. LOS ANGELES Monoclonal antibodies made headlines in early October when President Donald Trump was treated for COVID-19 with an experimental monoclonal antibody drug made by RegeneronAs part of a nationwide effort to discover more about the effects of this kind of therapy Keck Medicine of USC is enrolling outpatient volunteers in a national p.
The two new therapies from drug companies Eli Lilly and Regeneron work by preventing the coronavirus from attaching to cell receptors such as in lungs which in turn prevents the virus from replicating and infecting healthy cells. Co-developed by Eli Lilly and biotech company AbCellera Bamlanivimab was the first monoclonal antibody for Covid-19 granted Emergency Use Authorization by. Your primary care provider may be able to refer you to receive an infusion through your health care plan.
CVS Health was selected today by the US. MAb treatment for COVID-19 is different from a COVID-19. The treatments which are delivered through IV infusions that require.
The monoclonal antibody treatment is currently authorized to treat mild to moderate COVID-19 in patients who are at high risk for progressing to a severe case of COVID-19 or hospitalization said. The antibody treatment a cocktail of the monoclonal antibodies casirivimab and imdevimab that is made by Regeneron Pharmaceuticals is designed to. This page is no longer active.
Los Angeles Top Doctors. New Clinical Trial will Test Combination Monoclonal Antibody Therapy for COVID-19 Custom Content by the Los Angeles Business Journal Thursday January 21. Monoclonal antibodies are lab-produced molecules that act as substitutes for the bodys own infection-fighting antibodies.
Irfan KhanLos Angeles Times via Getty Images Monoclonal antibody drugs are supposed to help people with mild to moderate COVID-19 avoid the hospital but it can be a. CVS Healths infusion business will participate in a pilot to administer a recently authorized monoclonal antibody treatment to prevent patients from getting seriously ill with Covid-19. Irfan Khan Los Angeles Times Unfortunately thats where the list of approved and authorized drugs.
Department of Health and Human Services HHS to be part of Operation Warp Speed to pilot the administration of a. To learn about the use of MAb therapy see NIHs COVID-19 Treatment Guidelines Panels Statement on the Emergency Use Authorizations of Anti-SARS-CoV-2 Monoclonal Antibodies for the Treatment of COVID-19. The treatments are available to.
You may have heard about monoclonal antibody therapy the type of treatment.
Ucla Health Administers Monoclonal Antibody Treatments To Covid 19 Patients Daily Bruin

Antibody Treatment To Help Keep High Risk Coronavirus Patients Out Of Hospitals Cbs Los Angeles

Monoclonal Antibodies Overburdened Hospitals Are Not Offering This Treatment Against Covid 19 The Washington Post

A Covid Treatment That Not Enough People Know About Pittsburgh Business Times

Monoclonal Antibodies Infuse Hope For Those With Covid 19 Connect North Mississippi Health Services

Monoclonal Therapy State Survivor Corps

Monoclonal Therapy State Survivor Corps

Fda Approves Dual Monoclonal Antibody Therapy For Some Covid 19 Cases Youtube

Monoclonal Therapy State Survivor Corps
Https Healthpolicy Duke Edu Sites Default Files 2021 02 Promising 20practices 20for 20promoting 20utilization 20of 20covid 19 20monoclonal 20antibody 20treatmentsv4 Pdf
Usc Enrolling For Phase 3 Clinical Trial To Test Covid 19 Monoclonal Antibody Treatment Keck School Of Medicine Of Usc

Monoclonal Antibodies Could Ease Record Covid Hospitalizations Why Are They Going Unused
Emergency Medical Services Mab Emergency Medical Services Agency

Monoclonal Therapy State Survivor Corps
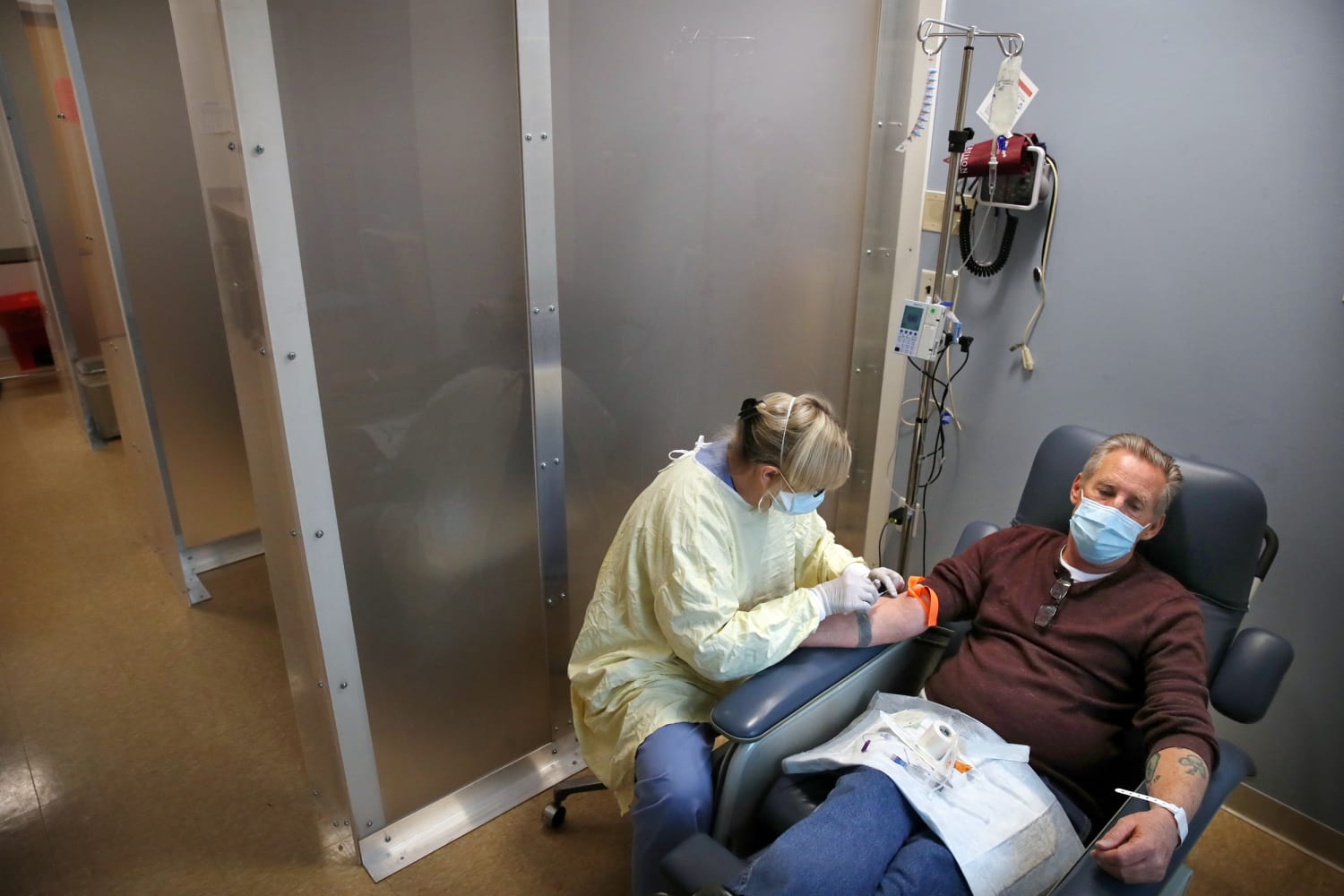
Monoclonal Antibodies Could Ease Record Covid Hospitalizations Why Are They Going Unused

Researchers Study Monoclonal Antibodies In Scid Treatment Immune Deficiency Foundation

Monoclonal Therapy State Survivor Corps


Post a Comment for "Monoclonal Antibody Treatment Los Angeles"